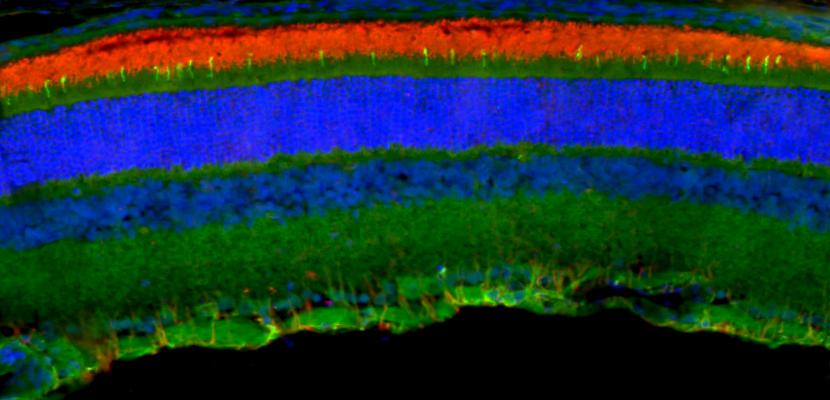
At the Clem Jones Centre for Regenerative Medicine, researchers combine their expertise in vision science and regenerative medicine to understand retinal diseases and explore the potential of stem cell therapies for tissue repair.
Our goal is to bring stem cell treatments to the clinic, offering hope for vision restoration in people with blinding retinal degenerative conditions like macular degeneration. Our team has developed several innovative procedures, now refined and in preparation for clinical trials.
In addition to retinal research, the Centre supports broader studies in regenerative medicine, stem cell biology, and vision science. Our work spans areas such as tissue engineering, tissue regeneration, biomaterials, advanced drug design and delivery, immunity and inflammation, and disease modelling.
Ultimately, the Centre’s mission is to translate breakthroughs in stem cell science into clinical treatments that directly benefit patients.