Researchers at the Clem Jones Centre for Regenerative Medicine are at the cutting edge of stem cell science, investigating the therapeutic use of stem cells in tissue repair.
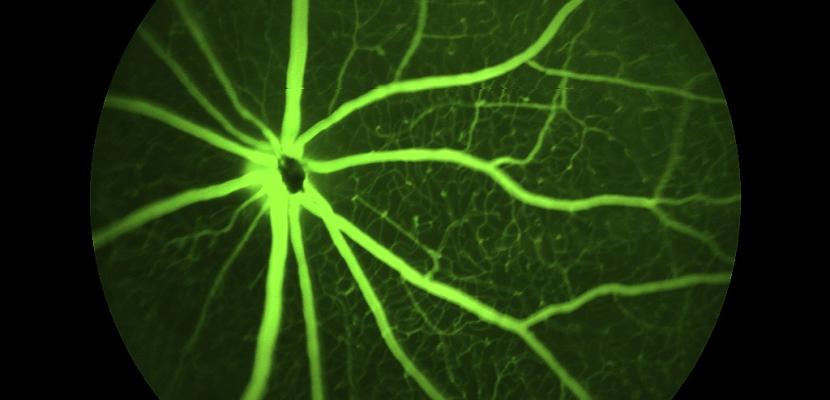
One of our significant long-term projects is the application of stem cell therapy to vision improvement for people suffering from macular degeneration. A number of ground-breaking procedures have been perfected by our team, in preparation for clinical trials.
The Centre also supports studies in the broader field of regenerative medicine and stem cell biology, extending to tissue engineering, tissue regeneration, biomaterials, intelligent drug delivery, immunity and inflammation, and advanced surgery.
Above all, the Centre’s goal is to translate research excellence in stem cell science to the clinical setting for the direct benefit of patients.